Abstract
Chromosomes fold in a hierarchy of increasingly complex structures from nucleosomes to chromosomal territories. Dynamic chromosomal architecture establishes regulatory cues that allow for local reorganization during cellular differentiation. The ring-shaped cohesin complex, composed of SMC1, SMC3, RAD21, and either STAG1 or STAG2, plays a critical role in forming chromatin loops, permitting genomic partitioning, enhancer-promoter interactions, and topological control of gene expression. While both STAG1 and STAG2 play a role in regulating gene expression and genome organization, we and others have defined distinct roles, owing to their differential cellular localization and range of interactions. Given that STAG2 is recurrently mutated in various cancers, including acute myeloid leukemia and myelodysplastic syndrome, understanding the role of STAG2 in gene regulation, hematopoiesis, and tumor suppression could provide novel therapeutic and clinical insights. Prior work by our group has demonstrated that Stag2 deficiency results in altered hematopoietic stem cell (HSC) self-renewal, impaired HSC differentiation, and lineage skewing. In particular, Stag2 deficiency resulted in marked features of myelodysplasia.
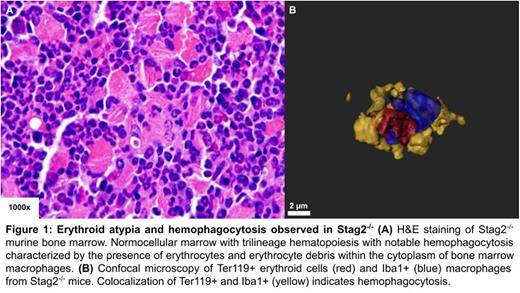
Here, we demonstrate that STAG2 loss results in erythroid atypia and hemophagocytosis. We observe decreased terminal erythroid maturation via scRNA-seq of Stag2-/- CFU-E (Lineage-cKit+Sca1−CD34−Fcγ−CD105+). To further investigate the mechanistic basis of this impaired maturation, bone marrow from Stag2-/-, Stag2+/-, and Stag2+/+ mice was stained with H&E, where marked nuclear-cytoplasmic dyssynchrony of erythroblasts was noted in a Stag2 dose dependent manner. Additionally, erythrocytes and erythrocyte debris was observed in bone marrow resident macrophages, suggesting the presence of hemophagocytosis (Fig. 1A). Immunohistochemistry (IHC) for Ter119, an erythroid specific protein, showed markedly reduced surface expression of Ter119, which was confirmed via flow cytometry (p < 0.001). In addition to the overall decrease in Ter119+ cells, the remaining IHC staining was concentrated into distinct foci with Stag2 loss. Immunohistochemistry for Iba1 showed an increased number of Iba1+ macrophages (Mφ) in Stag2-/- mice. Colocalization studies by confocal microscopy further validated hemophagocytosis (Fig.1B). To determine if Mφ overactivation was driving hemophagocytosis, Iba1+ Mφ from Stag2+/+ and Stag2-/- mice were stimulated with IL-4, IL-6, IFN-γ, and LPS and no differences observed as a function of Stag2 genotype. As no anemia present in Stag2-/- mice, we assayed serum erythropoietin and identified a significant increase in erythropoietin in Stag2-/- mice (p < 0.05), suggesting compensatory erythropoietic stimulation.
Flow cytometric analysis of Stag2+/+ and Stag2-/- bone marrow stained for CD44 showed significant alterations to terminal erythroid differentiation, with an increase in Stage II erythroblasts (p = 0.01) and decreases in Stage IV/V erythroblasts (p = 0.029 and 0.031, respectively). Subsequent ATAC sequencing of sorted Stag2+/+ and Stag2-/- erythroid progenitors showed an overall more relaxed chromatin state and hyperaccessibility with Stag2 loss, suggesting defective nuclear condensation from the earliest stages of erythropoiesis. Preliminary data from STAG2 shRNA knockdown in human CD34+ cells in vitro suggests that STAG2 may impair chromatin condensation and enucleation during terminal erythroid differentiation. Whether the hyperaccessibility observed with STAG2 loss persists through terminal erythroid differentiation is being investigated to determine the mechanism by which STAG2 loss influences defective nuclear condensation and nuclear extrusion during erythroid differentiation. These data suggest that Stag2 loss negatively affects chromatin condensation, resulting in a compensatory erythropoietin secretion and increased hemophagocytosis. These data extend the role of Stag2 from lineage priming to the latest stages of erythropoiesis and define the critical physiologic role of Stag2 in erythropoiesis.
Disclosures
Viny:Arima Genomics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Nooma Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal